Thermodynamic Database
Components (27)
A total of 27 components are included in the database as listed here:
Al-B-C-Co-Cr-Cu-Fe-Hf-Ir-Mn-Mo-N-Nb-Ni-O-P-Pt-Re-Ru-S-Si-Ta-Ti-V-W-Y-Zr
Suggested Composition Range
The suggested composition range for each element is listed in Table 1. It should be noted that this composition range is based on the validation we performed on commercial alloys. For particular subsystems, the application range may be wider. Some subsystems can be applied to the entire composition range as given in Section Assessed Subsystems.
| Elements | Composition Range (wt.%) |
|---|---|
|
Ni |
50-100 |
|
Pt |
0-40 |
|
Al,Co,Cr,Fe |
0-22 |
|
Ir,Mo,Re,Ru,Ta,V,W |
0-12 |
|
Hf,Nb,Ti |
0-5 |
|
B,C,Cu,Mn,N,O,Si,Y,Zr |
0-0.5 |
|
P,S |
0-0.01 |
Phases
Total of 343 phases are included in the current database. The names and thermodynamic models of some phases are given in Table 2. Information on all the other phases is listed in PanNi2024: List of Phases. Users can also view it through TDB viewer of Pandat™ .
Assessed Subsystems
A total of 310 subsystems, including 241 binary and 69 ternary subsystems have been assessed. The modeling status is indicated by numbers. The systems with number 10 are fully assessed in the whole composition range. The higher value shows higher reliability of the system.
Binary Systems (241)
| Al-B(10) | Al-C(10) | Al-Co(10) | Al-Cr(10) | Al-Hf(10) | Al-Ir(10) |
| Al-Mn(10) | Al-Nb(10) | Al-Ni(10) | Al-O(10) | Al-Pt(10) | Al-Re(10) |
| Al-Ru(10) | Al-S(10) | Al-Si(10) | Al-Ta(10) | Al-Ti(10) | Al-V(10) |
| Al-Y(10) | Al-Zr(10) | B-Co(10) | B-Cr(10) | B-Fe(10) | B-Hf(10) |
| B-Mo(10) | B-Nb(10) | B-Ni(10) | B-O(10) | B-Re(10) | B-Ta(10) |
| B-Ti(10) | B-V(10) | B-W(10) | B-Zr(10) | C-Cr(10) | C-Fe(10) |
| C-Hf(10) | C-Mo(10) | C-Nb(10) | C-Ni(10) | C-Ta(10) | C-Ti(10) |
| C-V(10) | C-W(10) | C-Zr(10) | Co-Cr(10) | Co-Cu(10) | Co-Fe(10) |
| Co-Hf(10) | Co-Mn(10) | Co-Mo(10) | Co-Nb(10) | Co-Ni(10) | Co-O(10) |
| Co-P(10) | Co-Si(10) | Co-Ta(10) | Co-Ti(10) | Co-V(10) | Co-W(10) |
| Co-Y(10) | Co-Zr(10) | Cr-Fe(10) | Cr-Cu(10) | Cr-Hf(10) | Cr-Ir(10) |
| Cr-Mn(10) | Cr-Mo(10) | Cr-N(10) | Cr-Nb(10) | Cr-Ni(10) | Cr-O(10) |
| Cr-P(10) | Cr-Pt(10) | Cr-Re(10) | Cr-Ru(10) | Cr-S(10) | Cr-Si(10) |
| Cr-Ta(10) | Cr-Ti(10) | Cr-V(10) | Cr-W(10) | Cr-Y(10) | Cr-Zr(10) |
| Cu-Fe(10) | Cu-Ir(10) | Cu-Mn(10) | Cu-Nb(10) | Cu-Ni(10) | Cu-O(10) |
| Cu-P(10) | Cu-Si(10) | Cu-Ta(10) | Cu-Ti(10) | Cu-V(10) | Cu-W(10) |
| Cu-Y(10) | Cu-Zr(10) | Fe-Hf(10) | Fe-Ir(10) | Fe-Mn(10) | Fe-Mo(10) |
| Fe-N(10) | Fe-Nb(10) | Fe-Ni(10) | Fe-O(10) | Fe-P(10) | Fe-Re(10) |
| Fe-S(10) | Fe-Si(10) | Fe-Ta(10) | Fe-Ti(10) | Fe-V(10) | Fe-W(10) |
| Fe-Y(10) | Fe-Zr(10) | Hf-Mn(10) | Hf-Mo(10) | Hf-N(10) | Hf-Nb(10) |
| Hf-Ni(10) | Hf-O(10) | Hf-Si(10) | Hf-Ta(10) | Hf-Ti(10) | Hf-V(10) |
| Hf-W(10) | Hf-Y(10) | Ir-Ni(10) | Ir-O(10) | Ir-Pt(10) | Ir-Y(10) |
| Mn-Mo(10) | Mn-Nb(10) | Mn-Ni(10) | Mn-O(10) | Mn-P(10) | Mn-Pt(10) |
| Mn-S(10) | Mn-Si(10) | Mn-Ta(10) | Mn-Ti(10) | Mn-V(10) | Mn-W(10) |
| Mn-Y(10) | Mn-Zr(10) | Mo-N(10) | Mo-Nb(10) | Mo-Ni(10) | Mo-O(10) |
| Mo-P(10) | Mo-Pt(10) | Mo-Re(10) | Mo-Ru(10) | Mo-S(10) | Mo-Si(10) |
| Mo-Ti(10) | Mo-V(10) | Mo-W(10) | Mo-Y(10) | Mo-Zr(10) | Mo-Zr(10) |
| N-Nb(10) | N-Ni(10) | N-Ta(10) | N-Ti(10) | N-V(10) | N-W(10) |
| N-Zr(10) | Nb-Ni(10) | Nb-O(10) | Nb-P(10) | Nb-Si(10) | Nb-Ti(10) |
| Nb-V(10) | Nb-W(10) | Nb-Y(10) | Nb-Zr(10) | Ni-O(10) | Ni-P(10) |
| Ni-Pt(10) | Ni-Re(10) | Ni-Ru(10) | Ni-S(10) | Ni-Si(10) | Ni-Ta(10) |
| Ni-Ti(10) | Ni-V(10) | Ni-W(10) | Ni-Y(10) | Ni-Zr(10) | O-P(10) |
| O-Pt(10) | O-Ru(10) | O-Si(10) | O-Ta(10) | O-Ti(10) | O-V(10) |
| O-W(10) | O-Y(10) | O-Zr(10) | P-Ti(10) | Pt-Si(10) | Pt-Ta(10) |
| Pt-V(10) | Pt-Y(10) | Pt-Zr(10) | Re-Si(10) | Re-Ta(10) | Re-W(10) |
| Re-Y(10) | Re-Zr(10) | Ru-Si(10) | Ru-Ta(10) | Ru-Y(10) | S-Ti(10) |
| Si-Ta(10) | Si-Ti(10) | Si-V(10) | Si-W(10) | Si-Y(10) | Si-Zr(10) |
| Ta-Ti(10) | Ta-V(10) | Ta-W(10) | Ta-Y(10) | Ta-Zr(10) | Ti-V(10) |
| Ti-W(10) | Ti-Y(10) | Ti-Zr(10) | V-W(10) | V-Y(10) | V-Zr(10) |
| W-Zr(10) | Y-Zr(10) | Al-Mo(8) | Al-Fe(6) | Al-W(6) | C-Re(6) |
| Co-Pt(6) | Fe-Pt(6) |
Ternary Systems (69)
| Al-Co-Ni(10) | Al-Cr-Ni(10) | Al-Co-W(10) | Al-Mo-Ni(8) | Al-Nb-Ni(8) | Al-Ni-Pt(8) |
| Al-Ni-Re(8) | Al-Ni-Si(8) | Al-Ni-Ta(8) | Al-Ni-Ti(8) | Al-Ni-W(8) | Co-Cr-Mo(8) |
| Co-Cr-Ni(8) | Co-Cr-Ti(8) | Co-Cr-W(8) | Co-Fe-W(8) | Co-Mo-Ni(8) | Co-Ni-Si(8) |
| Co-Ni-Ta(8) | Co-Ni-Ti(8) | Co-Ni-W(8) | Cr-Fe-Ni(8) | Cr-Fe-Ti(8) | Cr-Mo-Nb(8) |
| Cr-Mo-Ni(8) | Cr-Nb-Ni(8) | Cr-Ni-Pt(8) | Cr-Ni-Ru(8) | Cr-Ni-Ta(8) | Cr-Ni-Ti(8) |
| Cr-Ni-W(8) | Cu-Hf-Ni(8) | Cu-Nb-Ni(8) | Cu-Ni-Si(8) | Cu-Ni-Ta(8) | Cu-Ni-Ti(8) |
| Cu-Ni-Zr(8) | Fe-Mo-Ni(8) | Fe-Nb-Ni(8) | Fe-Ni-Si(8) | Fe-Ni-W(8) | Mo-Nb-Ni(8) |
| Mo-Ni-Si(8) | Mo-Ni-Ta(8) | Mo-Ni-W(8) | Nb-Ni-Ti(8) | Ni-Si-Ti(8) | Ni-Ti-W(8) |
| Al-B-Cr(6) | Al-B-Ni(6) | B-Co-Cr(6) | B-Co-Ni(6) | B-Cr-Fe(6) | B-Cr-Ti(6) |
| B-Fe-Ni(6) | B-Ni-Ti(6) | B-Ni-W(6) | C-Co-Cr(6) | C-Co-Fe(6) | C-Co-Ni(6) |
| C-Co-W(6) | C-Cr-Ni(6) | C-Cr-Ti(6) | C-Fe-Ni(6) | C-Fe-W(6) | C-Mo-Ti(6) |
| C-Mo-W(6) | C-Nb-W(6) | C-Ni-W(6) |
Database Validation
This database focuses on the nickel-rich corner of multicomponent nickel alloys and has been extensively tested and validated by a large number of commercial nickel alloys. Table 3 lists the alloys and references used for testing the current database. In the table, Tl, Ts, and Tγ' represent liquidus(γ starts to form from liquid), solidus (all liquid disappears), and γ' solvus (γ' starts to precipitate in the γ phase matrix) temperatures, respectively. fγ' represents the volume fraction of the γ' phase, and x(X) in γ , x(X) in γ' represent the equilibrium compositions of component X in the γ and γ' phases, respectively. The recommended composition ranges given in Table 1 are the ones that have been extensively tested. Users need to be careful when using the database beyond the suggested ranges.
This database can be used to calculate phase equilibria for multi-component alloys, such as the equilibrium between γ and γ'. It can be used to predict phase transformation temperatures, such as liquidus, solidus and γ' solvus. The fraction of each phase as a function of temperature and partitioning of components in different phases can also be calculated. In addition to equilibrium calculations, Scheil simulations can also be carried out using this database. Some calculation results are presented in Figure 1 to Figure 10.
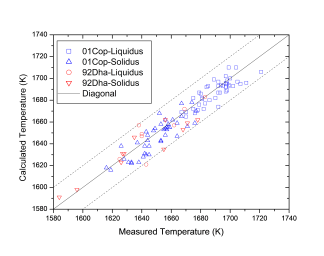
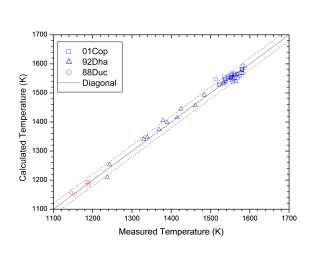
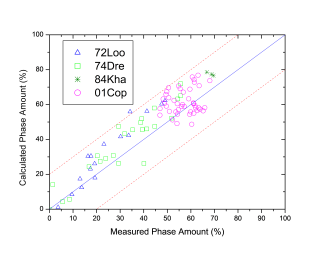
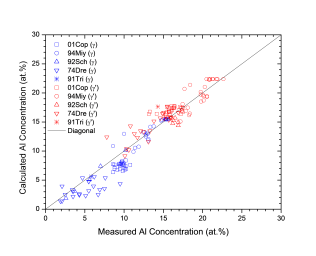
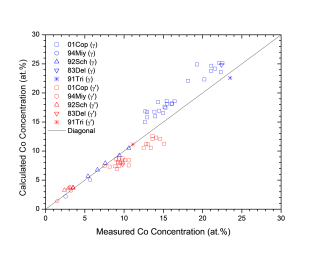
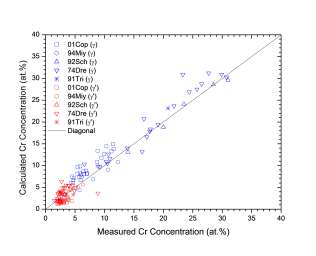
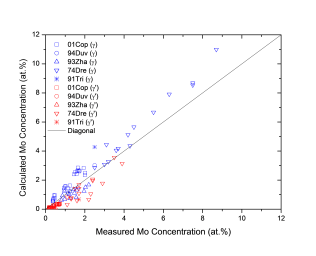
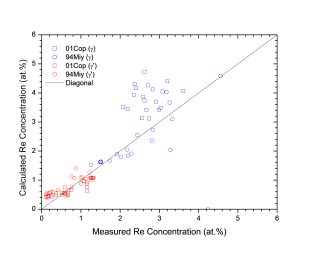
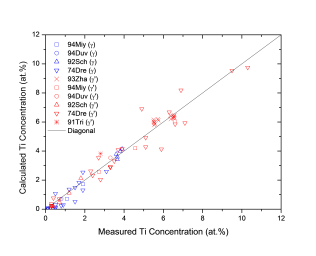
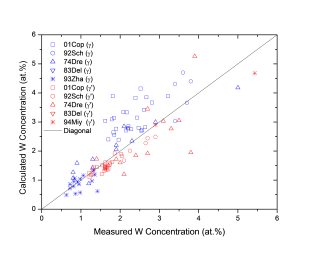
Figure 1 shows comparison between calculated and experimentally measured liquidus and solidus temperatures for nickel-based superalloys, while Figure 2 is for that of the solvus temperatures of the γ' phase. Figure 3 shows comparison between calculated and experimentally determined amounts of the γ' phase. Figure 4 to Figure 10 are comparisons between the calculated and experimentally measured equilibrium compositions for Al, Co, Cr, Mo, Re, Ti, and W in γ and γ' phases, respectively. These figures show reasonable agreement between the calculated values using the current nickel database and the experimentally determined ones.
Figure 1: Comparison between the calculated and experimentally measured liquidus and solidus temperatures
Figure 2: Comparison between the calculated and experimentally measured solvus temperatures of the γ' phase
Figure 4: Comparison between the calculated and experimentally measured equilibrium compositions of Al in γ and γ' phase
Figure 5: Comparison between the calculated and experimentally measured equilibrium compositions of Co in γ and γ' phases
Figure 6: Comparison between the calculated and experimentally measured equilibrium compositions of Cr in γ and γ' phases
Figure 7: Comparison between the calculated and experimentally measured equilibrium compositions of Mo in γ and γ' phases
Figure 8: Comparison between the calculated and experimentally measured equilibrium compositions of Re in γ and γ' phases
Figure 9: Comparison between the calculated and experimentally measured equilibrium compositions of Ti in γ and γ' phases
Figure 10: Comparison between the calculated and experimentally measured equilibrium compositions of W in γ and γ' phases
[1971Mol] E. H. van der Molen, J. M. Oblak and O. H. Kriege, Metal. Trans. 2 (1971): 1627-1633.
[1972Loo] W. T. Loomis, J. W. Freeman and D. L. Sponseller, Metal. Trans. 3 (1972): 989-1000.
[1974Dre] R. L. Dreshfield and J. F. Wallace, Metal. Trans. 5 (1974): 71-78.
[1983Del] K. M. Delargy and G. D. W. Smith, Metal. Trans. 14A (1983): 1771-1783.
[1983Mag] M. Magrini, B. Badan and E. Ramous, Z. Metallkde. 74 (1983): 314-316.
[1988Duc] C. Ducrocq, A. Lasalmonie and Y. Honnorat, in: S. Reichman, D. N. Duhl, G. Maurer, S. Antolovich and C. Lund (Eds.), Superalloys, The Metallurgical Society, 1988: 63-72.
[1991Tri] K. Trinckauf and E. Nembach, Acta Metall. Mater. 39(12) (1991): 3057-3061.
[1992Dha] S.-R. Dharwadkar, K. Hilpert, F. Schubert and V. Venugopal, Z. Metallkde. 83 (1992): 744-749.
[1992Sch] R. Schmidt and M. Feller-Kniepmeier, Scripta Metal. Mater. 26 (1992): 1919-1924.
[1993Zha] J. S. Zhang, Z.Q. Hu, Y. Murata, M. Morinaga and N. Yukawa, Metal. Trans. 24A (1993): 2443-2450.
[1994Duv] S. Duval, S. Chambreland, P. Caron and D. Blavette, Acta Metall. Mater. 42(1) (1994): 185-194.
[1994Miy] S. Miyazaki, Y. Murata and M. Morinaga, Iron and Steel (Japan) 80(2) (1994): 78-83.
[1998Rit] F. J. Ritzert, D. Arenas, D. Keller and V. Vasudevan, NASA/TM 1998-206622.
[1999Rit] F. J. Ritzert, D. Keller and V. Vasudevan, NASA/TM 1999-209277.
[2001Cop] E. H. Copland, N. S. Jacobson and F. J. Ritzert, NASA/TM 2001-210897.